After a government minister called on Liberians to “step up” and volunteer to test a new Ebola vaccine, angry callers on talk radio asked why no high-ranking government official had gotten a shot in the arm.
A local radio reporter asked whether signing a consent form was tantamount to a “death warrant” for volunteers. A daily newspaper said simply, “Liberians are not animals.”
West Africa’s Ebola epidemic may be waning, but another one in the future is a near certainty, health officials say.
Now, the United States is helping to lead a large study of two vaccines against Ebola. But as researchers try to compress a clinical process that can take a decade into a fraction of the time, they are confronting the same volatile mix of skepticism, fear, false rumor and understandable mistrust that helped spread Ebola in the first place.
Trials of Ebola drugs and vaccines are underway or planned in Liberia, Guinea and Sierra Leone, the three countries most affected by an epidemic that has claimed about 10,000 lives. But the study in Liberia of the two vaccines is the most ambitious, with American researchers from the National Institutes of Healthand their Liberian counterparts hoping to enlist more than 27,000 participants under an agreement between their governments.
The trial’s scale alone has posed tough ethical and practical questions. American and Liberian officials have debated how to attract so many volunteers, how much to pay them and how to mobilize the public to extinguish crippling rumors before they take root, like the one asserting that Ebola vaccines were being slipped into children’s immunizations.
And there is added layer of mistrust, directed at one of the most important partners in the trial: the Liberian government.
“This concept of social mobilization, I had not heard that term before,” said Dr. Clifford Lane, who is leading the trial for the National Institute of Allergy and Infectious Diseases, the American government research agency. “But I came to realize it is one of the most critical things for success in this country.”
Last August, even as Monrovia was rapidly becoming the center of the outbreak, many Liberians denied Ebola’s existence. Distrustful of a government widely perceived as corrupt, they believed that the authorities were exaggerating the gravity of the disease to get money from international donors. This made it harder to convince people to take lifesaving precautions like isolating sick relatives.
The distrust only deepened after the government deployed troops to enforce a blanket quarantine on a neighborhood in the capital, Monrovia, leading to deadly riots over a tactic that President Ellen Johnson Sirleaf later called a mistake.
“There were some false starts, but we built on the lessons from the false starts,” Dr. Stephen Kennedy, the lead Liberian investigator in the vaccine trial, said of last year’s efforts to combat the epidemic. “The lesson was that using formal and informal community structures had an impact on the epidemic in Liberia. So for this trial, we are building on that.”
To try to allay suspicions, Dr. Kennedy and another Liberian doctor invited the local news media to watch them get vaccinated. But the event did not appear to change the opinion of many Liberians, who continued to assert that their government was infecting citizens with Ebola to squeeze money out of donors.
The vaccine trials began taking shape after the Liberian government, at the height of the epidemic last year, asked the United States to conduct clinical research in Liberia on potential vaccines and drugs. Two vaccines, one manufactured by Merck and another by GlaxoSmithKline, were chosen after initial studies showed they were generally safe and produced an immune response against Ebola in human volunteers in the United States and other countries.

The West African trials are the first time vaccines are being tested in the context of an outbreak, though the waning caseload may make it more difficult to answer the ultimate question — whether they really protect people from contracting Ebola.
In the Liberian trial, which is expected to last about a year, participants will be given one of the two vaccines, or a placebo, at 10 locations in and around Monrovia.
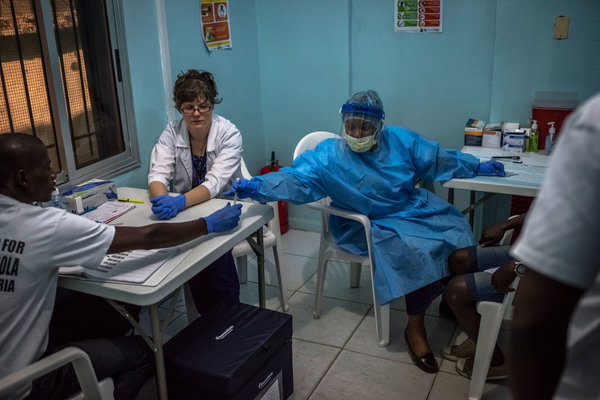
On Tuesday, researchers finished vaccinating an initial batch of 600 volunteers at Redemption Hospital, which was used as an Ebola holding center a few months ago. Officials said they had made sure that participants fully understood the consent forms — a critical issue in places with high illiteracy and low education levels, as in Liberia. Explanations of risks and potentially unfamiliar concepts, like placebos and randomized trials, are given in Liberian English, or, if need be, in one of the 16 local languages.
For 10 visits over the course of almost a year, the first 600 participants will each be handed $300: $40 on the first visit, less on subsequent visits, and $150 at the end. The money is supposed to compensate participants for transportation costs and lost wages.
“The ethics of it is compensation for inconvenience,” said Dr. Lane, the lead American researcher. “You don’t pay people to be part of medical research.”
Some of the American researchers have argued that the $300 compensation – the equivalent of $30 for each visit to the hospital – is too high, especially in a city where many people earn less than $5 a day. One Liberian newspaper announced the first hospital visit’s compensation in huge print on its front page: “US$40.”
“Businesses abuse labor because people don’t have a choice,” he said.
In Guinea, where Ebola cases continue, a trial began last weekend with the vaccination of 22 volunteers, including the country’s health minister, the head of the Guinean Red Cross and the president of the country’s Ebola research commission.
“They wanted to show by example to the population that they were willing to step forward and take the vaccine,” said Dr. Marie-Paule Kieny, an assistant director general at the World Health Organizationwho also volunteered for the study.

Braving Ebola
In Sierra Leone, which is also wrestling with Ebola cases, study co-sponsors, including the federalCenters for Disease Control and Prevention and the country’s Health Ministry, are hoping to avoid the opposition and misconceptions experienced in Liberia.
Vaccine experts have made presentations to Parliament and at gatherings of local chiefs. The phrase “Ebola prevention vaccine” is used rather than “Ebola vaccine,” to help avoid the impression that the vaccine might cause Ebola. And the study’s original acronym, Sleves, was discarded over concerns that it might remind residents of an ominous chapter in the country’s history: the civil war, when long or short “sleeves” often referred to hacking off a victim’s hands or arms.
Intended to enroll 6,000 to 8,000 volunteers, Sierra Leone’s study will focus on health professionals, disease surveillance officers, ambulance teams and other front-line staff members at risk of contracting Ebola. It will not include placebos and is awaiting final approval from the country’s pharmacy board.
Abbas Koroma, an environmental health officer in Sierra Leone, said that some controversy over the vaccines was to be expected in a democracy, but that the trial would be historic.
“I will tell my grandchildren we pioneered this,” he said.
For Liberia’s trials, Joseph Boye Cooper, a Liberian working for the effort, has gone out to neighborhoods, answering questions, allaying fears and recruiting volunteers. Mr. Cooper, who was a leader in a large volunteer Ebola watchdog group last year, uses the word “study” instead of “trial,” which he said invariably caused listeners to ask defensively, “Why do you want to try this vaccine on me?”
Before visiting neighborhoods, Mr. Cooper is careful to park the team’s sport utility vehicle several blocks away and take a motorcycle taxi to his destination. Arriving in a big car would merely fuel popular suspicions about government waste and corruption, he said.
“They’ll say I’m eating Ebola money and I must share some with them,” he said.
After one meeting inside a church, about half of the 24 listeners gave Mr. Cooper their names and cellphone numbers. The next morning, at 7 a.m., Mr. Cooper stood along a main road, waiting for the volunteers. With nobody after 20 minutes, he took out his cellphone.
“You just getting up, oh?” he said to one person, adding, with feeling: “I know. So how long will it take for you to get ready?” Eventually, about a dozen showed up, including people from the day before.
Samuel Weah, who had been convinced after listening to Mr. Cooper, said he had unsuccessfully tried to get family and friends to join him.
“They said they want more Ebola patients because government is using them to make money,” he said. “The more dead and infections, the more money.”
“But,” he added, “some said I should go and take the lead first. They said if nothing happens to me, they might come next.”